To prepare nimodipine nanosuspension, the study employed media milling, a high-efficiency and reproducible method. Nimodipine is a second-generation calcium channel blocker used to treat ischemic neurological conditions resulting from brain blood vessel spasms. However, nimodipine has low solubility in water and poor bioavailability, making it challenging to develop an effective formulation.
Nanosuspensions are stable colloidal systems in which drug nanoparticles are dispersed in water using stabilizers. They have the advantage of significantly enhancing drug saturation solubility, improving dissolution rates, and ultimately increasing bioavailability. Common methods for preparing nanosuspensions include precipitation, high-pressure homogenization, and media milling, with media milling being widely used due to its high production efficiency, strong controllability, and excellent reproducibility.
In this study, the media milling technique was employed to prepare nimodipine nanosuspension, and the process was optimized based on particle size and zeta potential. Several key factors, including stabilizer concentration, the ratio of stabilizers, drug concentration, milling speed, milling time, and type of zirconia beads, were examined to optimize the process.
Equipment and Materials:
The following equipment and materials were used:
DYNO-Mill Research Lab media milling machine
NanoZS-90 laser particle size analyzer
Waters 1525 high-performance liquid chromatography system
Lyo-0.5L freeze dryer
Netzsch 204F1 differential scanning calorimeter
Bruker D8Advance powder X-ray diffractometer
Hitachi H-600 transmission electron microscope
Nimodipine raw material
Reference standard for content determination
Sodium dodecyl sulfate (SDS)
Poloxamer 188 (F68)
Hydroxypropyl methylcellulose E5 (HPMC E5)
Methods and Results:
Nanosuspension Preparation: Stabilizers were added to 100 mL of distilled water and sonicated to dissolve completely. Nimodipine (average particle size of 5 μm) was slowly added under stirring at 100 rpm for 10 minutes, forming the initial suspension. The suspension was then milled in the media milling machine.
Particle Size and Zeta Potential Measurement: The particle size, polydispersity index (PDI), and zeta potential of the nanosuspension were measured using a laser particle size analyzer.
Single-Factor Experiments for Optimization:
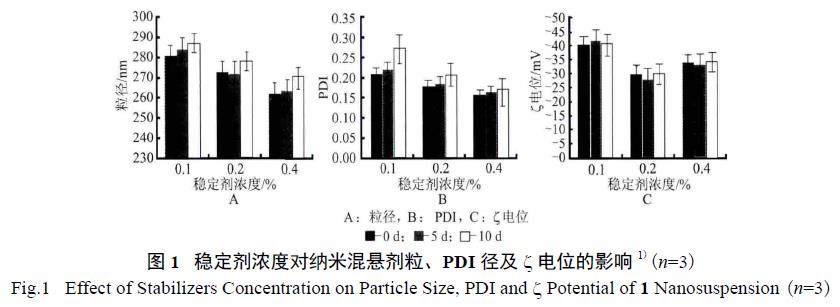
3.1. Effect of Stabilizer Concentration: Various concentrations of a stabilizer mixture (F68 and HPMC) were used, and their impact on particle size, PDI, and stability was assessed. The study determined that a stabilizer concentration of 0.4% was optimal.
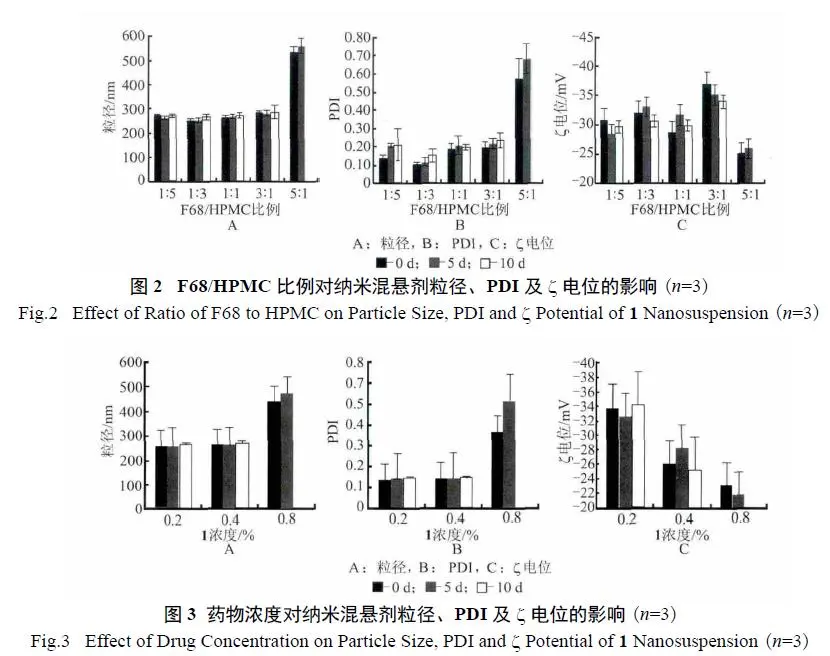
3.2. Effect of F68/HPMC Ratio: Different mass ratios of F68 and HPMC were examined, with the 1:3 ratio found to be optimal.
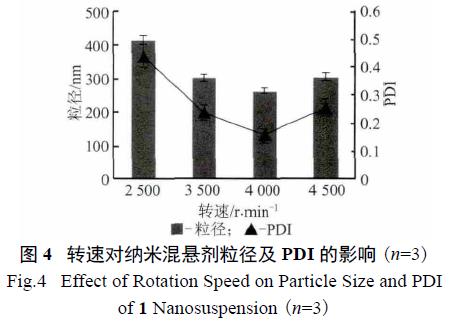
3.3. Effect of Drug Concentration: Different nimodipine concentrations were tested, and it was found that a concentration of 0.2% yielded the best results.
Optimization of Process Parameters:
4.1. Zirconia Bead Size: Two zirconia bead sizes (0.3 mm and 0.1 mm) were tested at a milling speed of 4000 rpm for 15 minutes. The results showed that 0.1 mm beads were more effective, resulting in a particle size of (295.6±13.4) nm.
4.2. Milling Speed: Various milling speeds (2500, 3500, 4000, and 4500 rpm) were examined at 0.1 mm bead size. A speed of 4000 rpm was chosen as it yielded the best results.
4.3. Milling Time: Different milling times (5, 10, 15, 30, and 45 minutes) were investigated at 4000 rpm. A milling time of 15 minutes was found to be optimal.
Process Reproducibility: Three batches of nimodipine nanosuspension were prepared according to the optimized parameters, and their particle size, PDI, and zeta potential were measured. The results showed good reproducibility.
Characterization:
6.1. Morphology of Nanosuspension: Transmission electron microscopy revealed that the nimodipine nanoparticles were nearly spherical with good dispersion and a particle size of approximately 250 nm.
6.2. Investigation of Nanosuspension Freeze-Dried Powder Crystal Form: X-ray diffraction (XRD) and differential scanning calorimetry (DSC) were conducted. XRD results indicated that the drug maintained its crystalline form after milling and drying. DSC results supported this finding, showing that the characteristic endothermic peak of the drug remained in the freeze-dried nanosuspension powder.
Saturation Solubility of Freeze-Dried Nanosuspension:
7.1. Chromatographic Method: A high-performance liquid chromatography system was used to establish a standard curve for nimodipine concentration in a suitable solvent system. The linearity, precision, and accuracy of the method were validated.
7.2. Determination of Saturation Solubility: Excess nimodipine and the freeze-dried nanosuspension were added to distilled water and shaken at room temperature. The saturation solubility of nimodipine in the freeze-dried nanosuspension was found to be nearly 60 times higher than that of the original drug.
Discussion:
Media milling is a highly efficient and reproducible method for preparing nanosuspensions. This study successfully optimized the parameters for nimodipine nanosuspension preparation, including stabilizer concentration, stabilizer ratio, drug concentration, bead size, milling speed, and milling time. The freeze-dried nanosuspension exhibited improved solubility compared to the original drug, confirming the relationship between particle size reduction and increased solubility.
In conclusion, the media milling method offers a practical and efficient approach to enhance the solubility and bioavailability of poorly water-soluble drugs, such as nimodipine, and has the potential for broader applications in the pharmaceutical industry.

Submit your demand,
we will contact you ASAP.

Sanxin New Materials Co., Ltd. focus on producing and selling ceramic beads and parts such as grinding media, blasting beads, bearing ball, structure part, ceramic wear-resistant liners, Nanoparticles Nano Powder

